For a complete list of publications, please visit Elisa’s GoogleScholar page.
Mouse Surgical Model of Mechanical Uterine Injury and Subsequent Embryo Defects
Elisa T. Zhang. Current Protocols. 2024.
Although C-sections represent ∼30% of deliveries in the US, the mechanisms by which uterine injury leads to pregnancy disorders such as placenta previa and placenta accreta are poorly understood. Animal models of uterine injury are necessary to gaining insights into these questions, as well as developing a platform for testing new strategies for diagnosis and intervention. Therefore, this article describes in detail a novel methodology for introducing uterine injury into mice and assessing its consequences on outcomes in utero.
Pubmed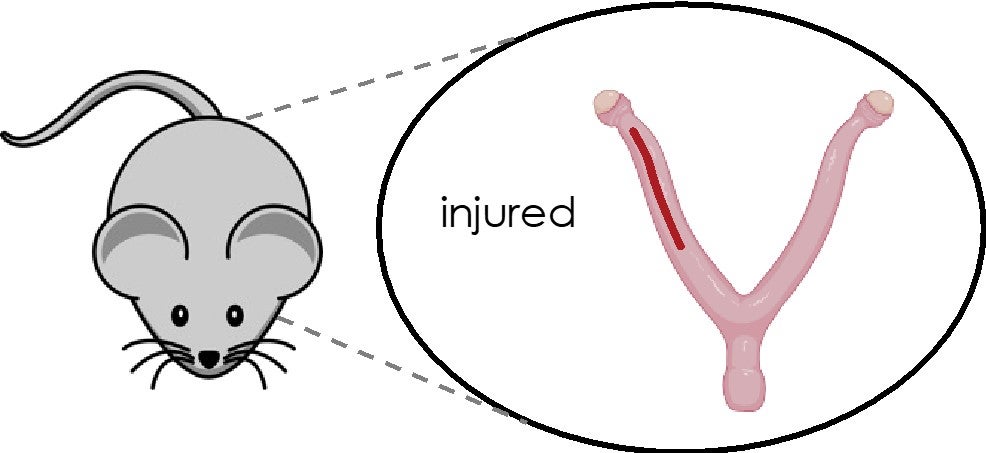
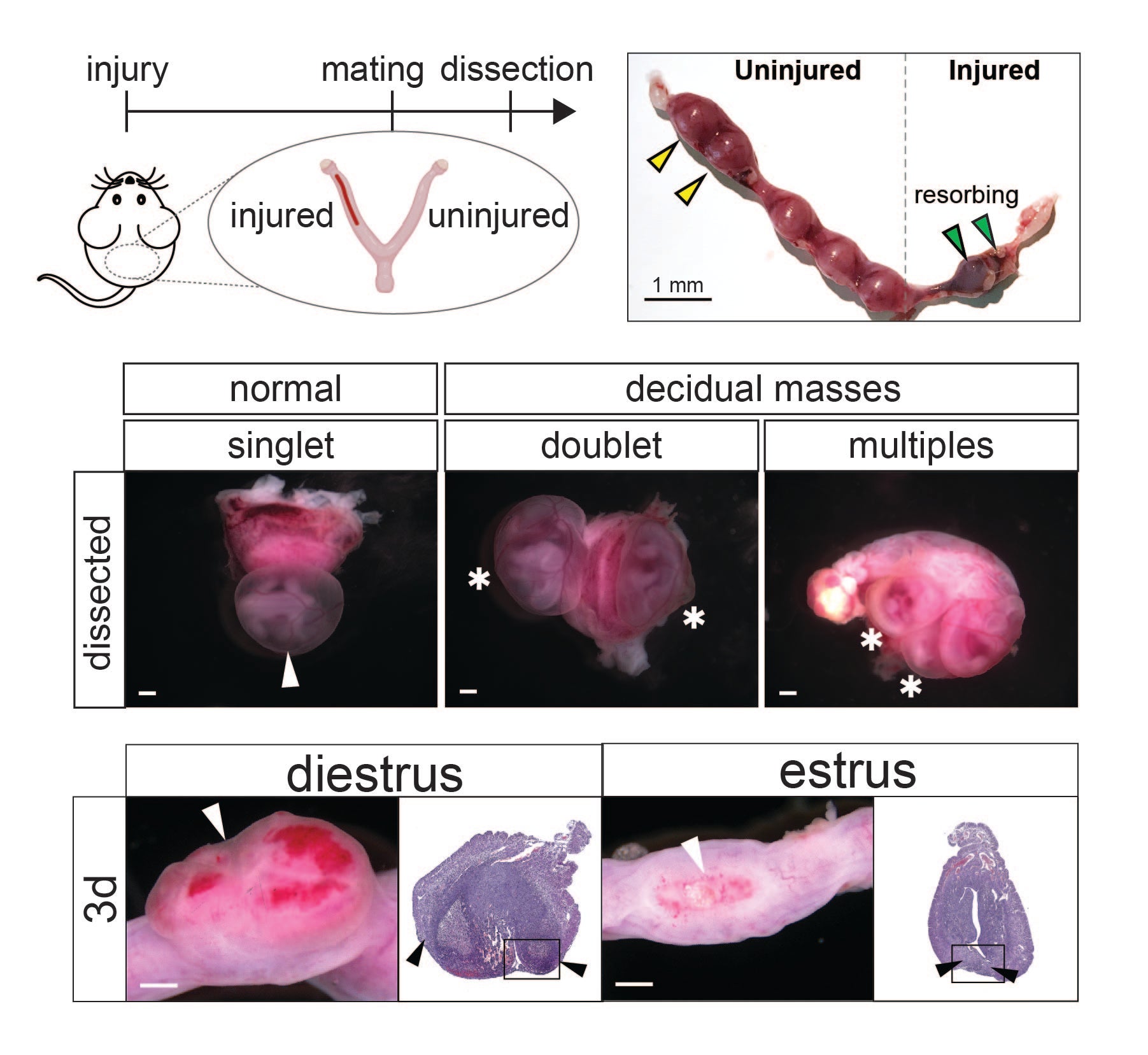
Uterine injury during diestrus leads to placental and embryonic defects in future pregnancies in mice
Elisa T. Zhang, Kristen L. Wells, Abby J. Bergman, Emily E. Ryan, Lars M. Steinmetz, Julie C. Baker. Biology of Reproduction. 2024.
While uterine injury from procedures such as C-section can lead to subsequent pregnancy disorders such as placenta previa, placenta accreta, and infertility, little is understood about the mechanistic links between uterine injury and subsequent pregnancy outcomes. To address this knowledge gap, we developed a novel rodent model of uterine injury to comprehensively assess subsequent in utero outcomes. We observed 3 outcomes with parallels to human disease: embryo resorption (infertility), embryo misspacing (placenta previa), and thinned decidua (placenta accreta). Intriguingly, mice subjected to injury during diestrus but not estrus exhibited embryo spacing defects. This led to delayed wound healing and perturbations in several COX/prostaglandin pathway genes, suggesting that uterine state dictates structural and molecular responses to injury and subsequent functionality, as well as opportunities for intervention at the time of injury to govern outcomes.
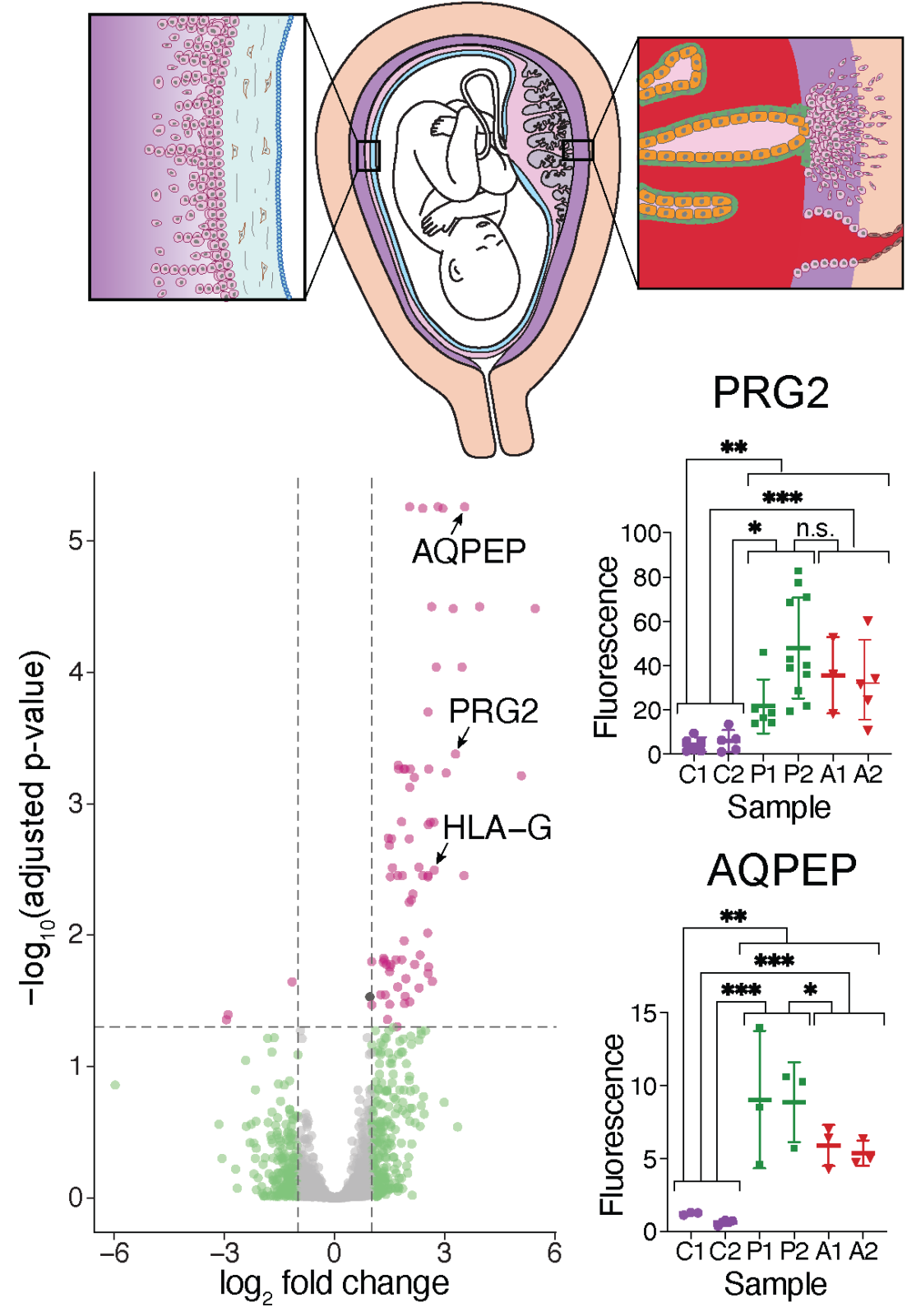
PRG2 and AQPEP are misexpressed in fetal membranes in placenta previa and percreta
Elisa T. Zhang, Roberta L. Hannibal, Keyla M. Badillo Rivera Janet H. T. Song, Kelly McGowan, Xiaowei Zhu, Gudrun Meinhardt, Martin Knöfler, Jürgen Pollheimer, Alexander E. Urban, Ann K. Folkins, Deirdre J. Lyell, Julie C Baker. Biology of Reproduction. 2021.
In this study, we find that human extraembryonic tissues surrounding the conceptus harbor a signature of previa and the accreta spectrum when compared to controls. This signature, including elevated protein levels of PRG2 and AQPEP, is characteristic of extravillous trophoblasts (EVTs) and may thus reflect increased trophoblast invasiveness in these pregnancy conditions.
A human autoimmune organoid model reveals IL-7 function in coeliac disease
António J. M. Santos, Vincent van Unen, Zhongqi Lin, Steven M. Chirieleison, Nhi Ha, Arpit Batish, Joshua E. Chan, Jose Cedano, Elisa T. Zhang, Qinghui Mu, Alexander Guh-Siesel, Madeline Tomaske, Deana Colburg, Sushama Varma, Shannon S. Choi, Asbjørn Christophersen, Ani Baghdasaryan, Kathryn E. Yost, Kasper Karlsson, Andrew Ha, Jing Li, Hongjie Dai, Zachary M. Sellers, Howard Y. Chang, James C. Y. Dunn, Bing M. Zhang, Elizabeth D. Mellins, Ludvig M. Sollid, Nielsen Q. Fernandez-Becker, Mark M. Davis & Calvin J. Kuo. Nature. 2024.
In this study, we demonstrated that air-liquid interface (ALI) organoids generated from intact fragments of human duodenum preserve epithelium alongside native mesenchyme and tissue-resident immune cells as a complete entity without reconstitution. Using these human duodenal ALI organoids derived from endoscopic biopsies of celiac disease patients, we examined the immune network response stimulated by gluten epitopes. Further functional studies revealed that interleukin-7 (IL-7) is induced by gluten to regulate CD8+ T cell activity and is necessary and sufficient for epithelial destruction. We then demonstrate that IL-7 is highly upregulated in the lamina propria mesenchyme in patient biopsies from active celiac disease compared to remission controls. Altogether, our human in vitro model of celiac disease enables mechanistic investigation and provides proof-of-principle for the use of human organoids as models of autoimmunity.
Publication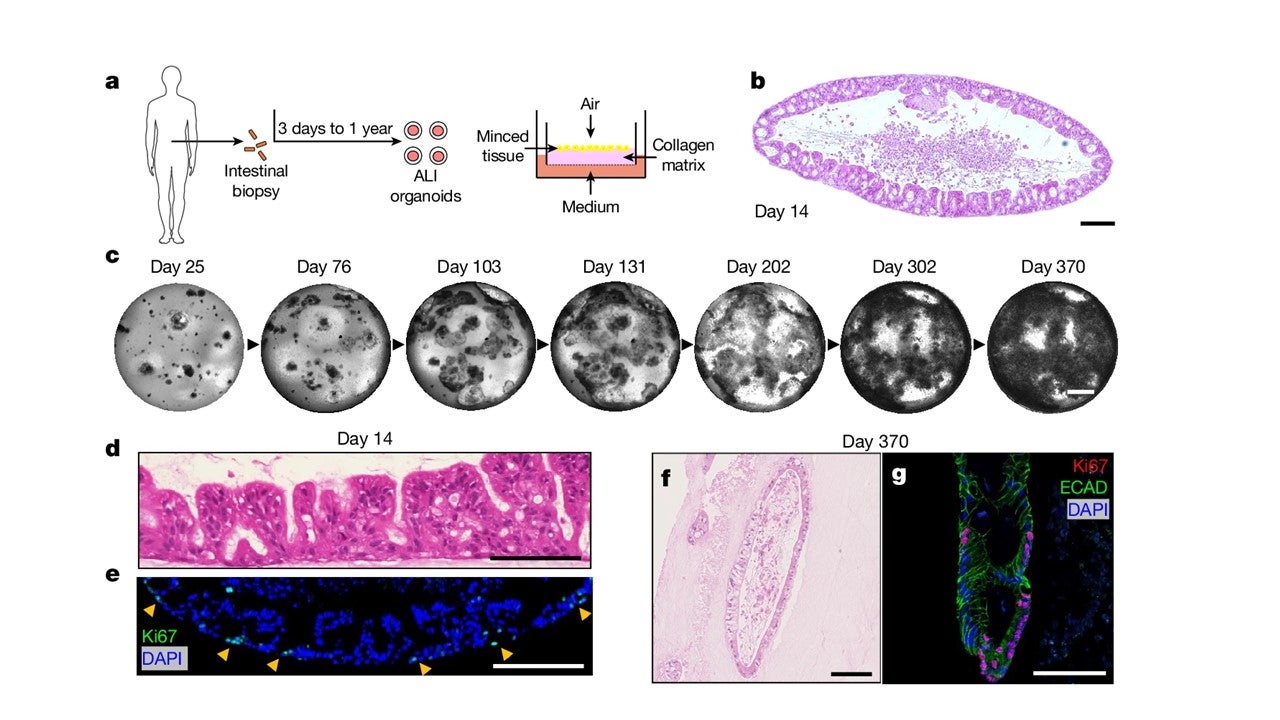
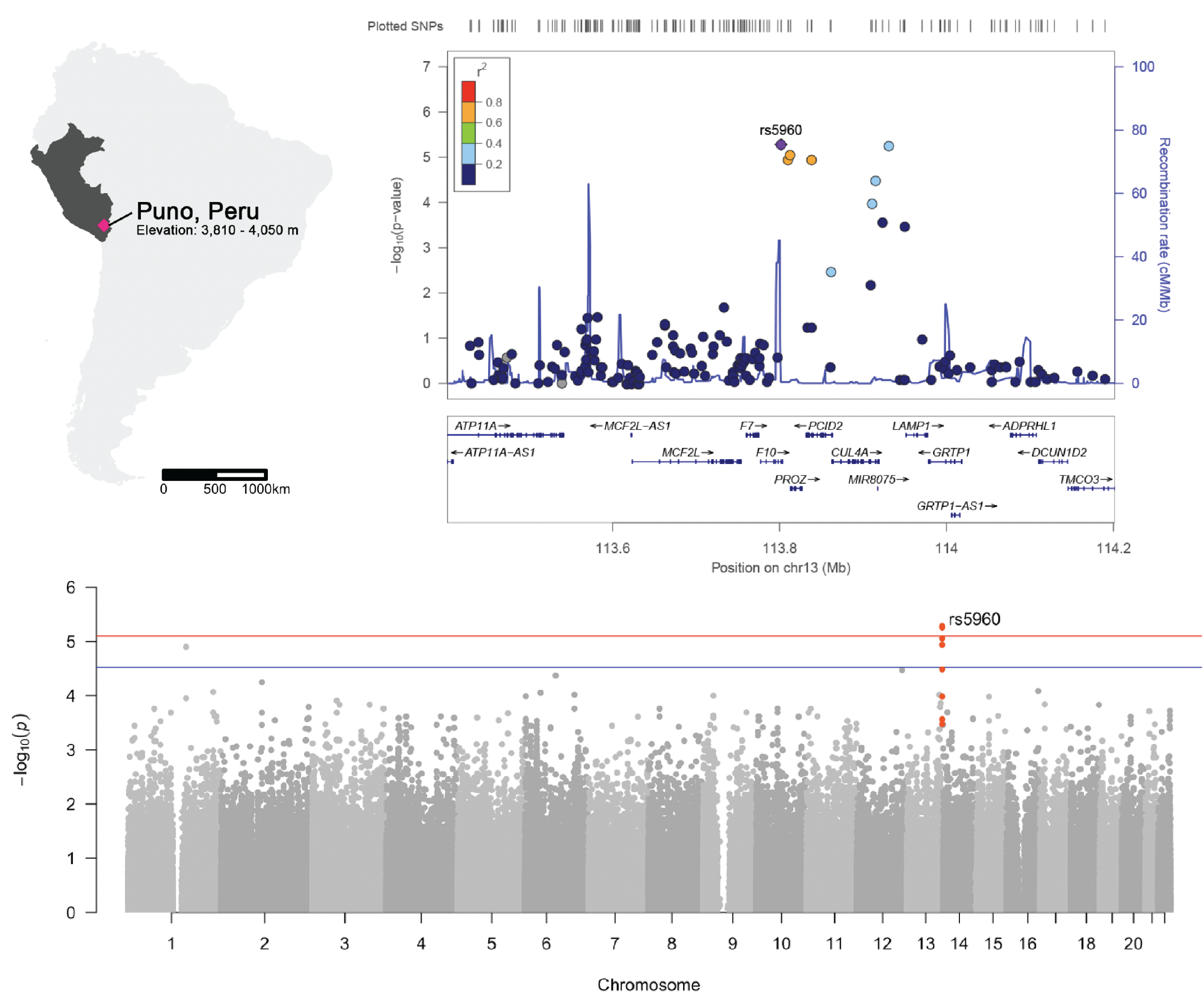
Clotting factor genes are associated with preeclampsia in high altitude pregnant women in the Peruvian Andes
Maria A. Nieves-Colón*, Keyla M. Badillo Rivera*, Karla Sandoval Mendoza, Vanessa Villanueva Dávalos, Luis E. Enriquez Lencinas, Javier Mendoza-Revilla, Kaustubh Adhikari, Ram González-Buenfil, Jessica W. Chen, Elisa T. Zhang, Alexandra Sockell, Patricia Ortiz Tello, Gloria M. Hurtado, Ramiro Condori Salas, Ricardo Cebrecos, José C. Manzaneda Choque, Franz P. Manzaneda Choque, Germán P. Yábar Pilco, Erin Rawls, Celeste Eng, Scott Huntsman, Esteban González Burchard, Andrés Ruiz-Linares, Rolando González-José, Gabriel Bedoya, Maria Cátira Bortolini, Giovanni Poletti, Carla Gallo, Francisco Rothhammer, Carlos D. Bustamante, Julie C. Baker, Christopher R. Gignoux, Genevieve L. Wojcik, Andrés Moreno-Estrada. American Journal of Human Genetics. 2022.
While preeclampsia is a major cause of morbidity and mortality for pregnant women and newborns worldwide, the heterogeneity of preeclampsia has posed a challenge to understanding its molecular basis. In this study, we investigate a cohort of preeclamptic Highland Andean families (N=883) from Puno, Peru—a city located 3,500 meters above sea level—to elucidate the genetic basis of preeclampsia at high altitudes. We identified strong associations with several variants near genes involved in placental and blood vessel function, and thus, of functional importance for human pregnancy biology. Our findings support the concept that coagulation plays an important role in the pathology of preeclampsia and potentially underlies other pregnancy disorders exacerbated at high altitude.
Los genes del factor de coagulación están asociados con la preeclampsia en mujeres embarazadas de altura en los Andes peruanos
Maria A. Nieves-Colón*, Keyla M. Badillo Rivera*, Karla Sandoval Mendoza, Vanessa Villanueva Dávalos, Luis E. Enriquez Lencinas, Javier Mendoza-Revilla, Kaustubh Adhikari, Ram González-Buenfil, Jessica W. Chen, Elisa T. Zhang, Alexandra Sockell, Patricia Ortiz Tello, Gloria M. Hurtado, Ramiro Condori Salas, Ricardo Cebrecos, José C. Manzaneda Choque, Franz P. Manzaneda Choque, Germán P. Yábar Pilco, Erin Rawls, Celeste Eng, Scott Huntsman, Esteban González Burchard, Andrés Ruiz-Linares, Rolando González-José, Gabriel Bedoya, Maria Cátira Bortolini, Giovanni Poletti, Carla Gallo, Francisco Rothhammer, Carlos D. Bustamante, Julie C. Baker, Christopher R. Gignoux, Genevieve L. Wojcik, Andrés Moreno-Estrada. American Journal of Human Genetics. 2022.
Si bien la preeclampsia es una causa importante de morbilidad y mortalidad en mujeres embarazadas y recién nacidos en todo el mundo, la heterogeneidad de la preeclampsia ha planteado un desafío para comprender su base molecular. En este estudio, investigamos una cohorte de familias andinas preeclámpticas (N=883) de Puno, Perú, una ciudad ubicada a 3500 metros sobre el nivel del mar, para dilucidar la base genética de la preeclampsia en altitudes elevadas. Identificamos fuertes asociaciones con varias variantes cerca de genes involucrados en la función de la placenta y los vasos sanguíneos y, por lo tanto, de importancia funcional para la biología del embarazo humano. Nuestros hallazgos respaldan el concepto de que la coagulación juega un papel importante en la patología de la preeclampsia y, potencialmente, es la base de otros trastornos del embarazo exacerbados a gran altura.

Architecture of the human XPC DNA repair and stem cell coactivator complex
Elisa T. Zhang, Yuan He, Patricia Grob, Yick W. Fong, Eva Nogales, and Robert Tjian. PNAS. 2015.
Embryonic or pluripotent stem cells are unique in their ability to self-renew in culture and to generate all lineages of an adult organism, thus providing a valuable window into early development and regenerative biology. The Xeroderma pigmentosum complementation group C (XPC) DNA repair complex is a key regulator of pluripotent gene expression. This study presents the first complete structures of different XPC complexes by electron microscopy, and combined with biochemical approaches, we synthesize a model of how XPC performs both its evolutionarily conserved DNA repair function and its nonconserved transcription function.
Pubmed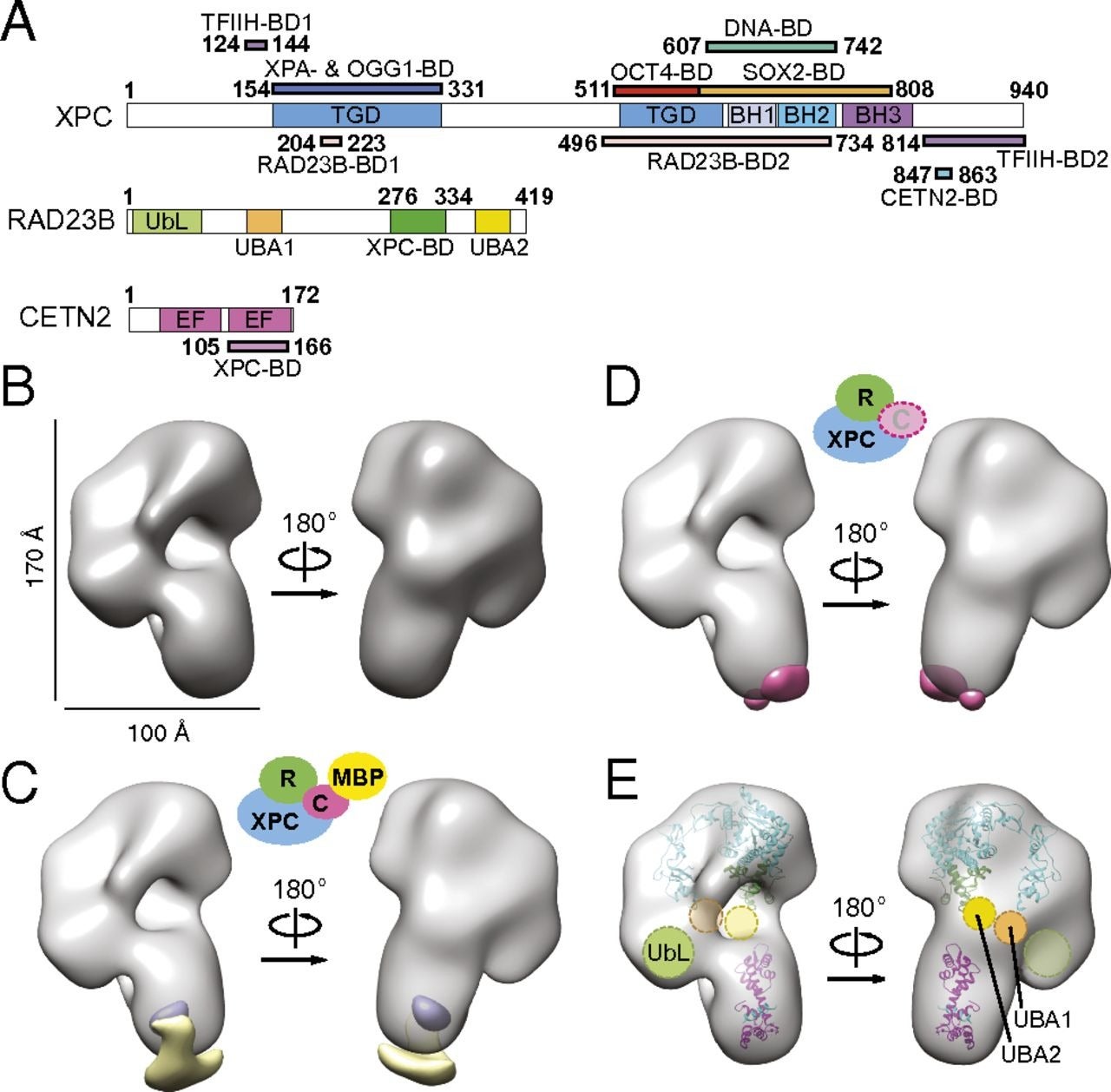
Functional and mechanistic studies of XPC DNA-repair complex as transcriptional coactivator in embryonic stem cells
Claudia Cattoglio, Elisa T. Zhang, Ivan Grubisic, Kunitoshi Chiba, Yick W. Fong, and Robert Tjian. PNAS. 2015.
The embryonic stem cell (ESC) state is transcriptionally controlled by OCT4, SOX2, and NANOG with cofactors, chromatin regulators, noncoding RNAs, and other effectors of signaling pathways. We recently identified the DNA-repair complex xeroderma pigmentosum C (XPC)-RAD23B-CETN2 as a stem cell coactivator (SCC) required for OCT4/SOX2 transcriptional activation. Here we investigate the role of SCC genome-wide in murine ESCs by mapping regions bound by RAD23B and analyzing transcriptional profiles of SCC-depleted ESCs. We establish OCT4 and SOX2 as the primary transcription factors recruiting the XPC complex to regulatory regions of pluripotency genes and identify the XPC subunit as essential for interaction with the two proteins. We further identify a novel RNA-mediated interaction between XPC and SOX2 that we subsequently identified as required for full transcriptional activity.
Pubmed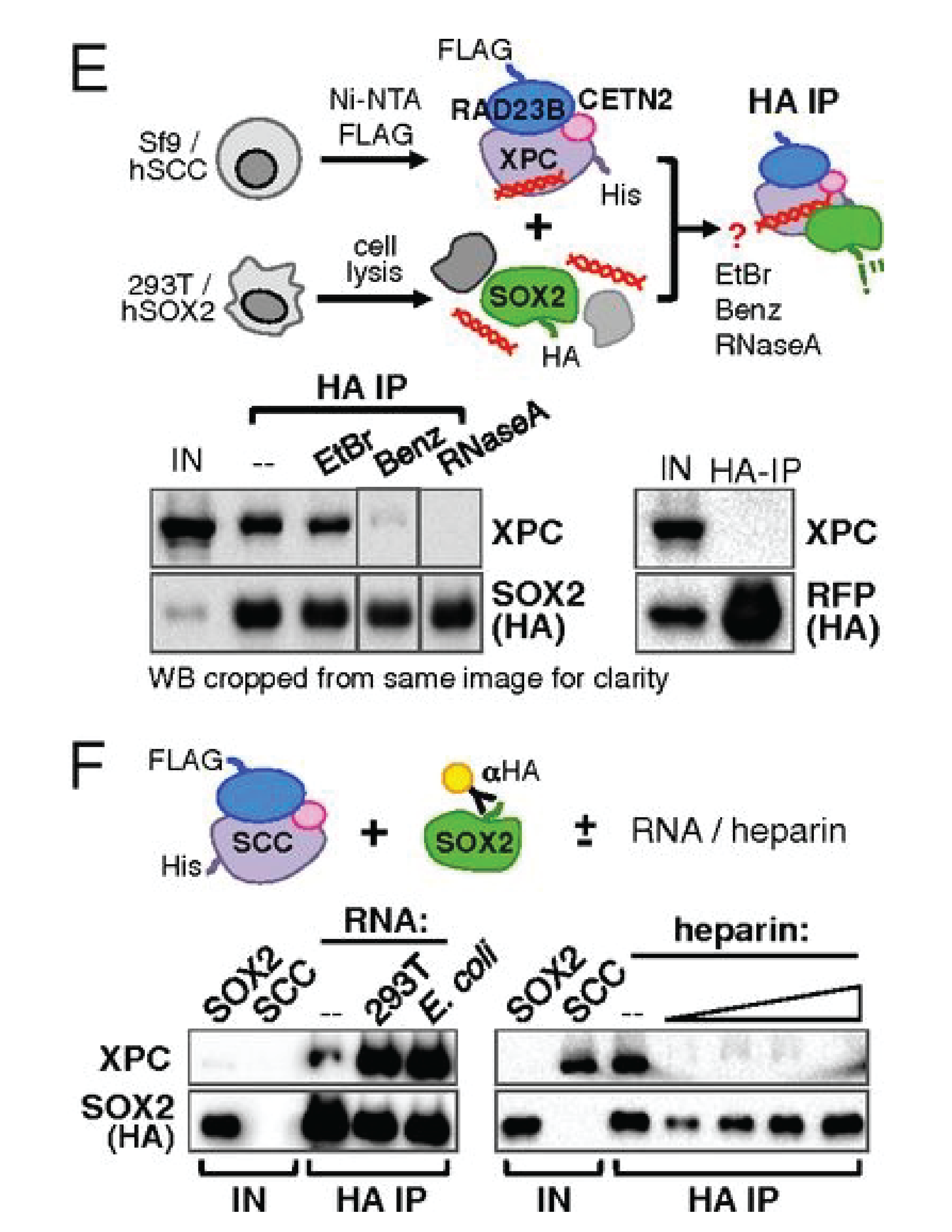
Dynamics of CRISPR-Cas9 genome interrogation in living cells
Spencer C. Knight, Lianqi Xie, Wulan Deng, Benjamin Guglielmi, Lea B. Witkowsky, Lana Bosanac, Elisa T. Zhang, Mohamedd El Beheiry, Jean-Baptiste Masson, Maxine Dahan, Zhe Liu, Jennifer A. Doudna, and Robert Tjian. Science. 2015.
The Cas9 nuclease forms the heart of the CRISPR-Cas genome editing system. Cas9 binds small guide RNAs that direct it to its target sites, where the nuclease either cleaves or binds to genomic DNA. Here we used single-molecule imaging to track Cas9 in living cells. Cas9 searches the genome for its target sites using rapid threedimensional diffusion. It spends very little time binding to off-target sites, which explains the high accuracy of the CRISPRCas9 editing machine.
Pubmed
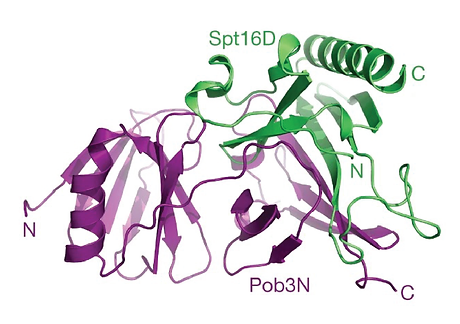
Structural basis of histone H2A–H2B recognition by the essential chaperone FACT
Maria Hondele*, Tobias Stuwe*, Markus Hassler, Felix Halbach, Andrew Bowman, Elisa T. Zhang, Bianca Nijmeijer, Christiane Kotthoff, Vladimir Rybin, Stefan Amlacher, Ed Hurt, and Andreas Ladurner. Nature 2013.
Facilitates chromatin transcription (FACT) is a conserved histone chaperone that reorganizes nucleosomes and ensures chromatin integrity during DNA transcription, replication and repair. Key to the broad functions of FACT is its recognition of histones H2A-H2B (ref. 2). However, the structural basis for how histones H2A-H2B are recognized and how this integrates with the other functions of FACT, including the recognition of histones H3-H4 and other nuclear factors, is unknown. Here we reveal the crystal structure of the evolutionarily conserved FACT chaperone domain Spt16M from Chaetomium thermophilum, in complex with the H2A-H2B heterodimer. A novel 'U-turn' motif scaffolded onto a Rtt106-like module embraces the α1 helix of H2B. Biochemical and in vivo assays validate the structure and dissect the contribution of histone tails and H3-H4 towards Spt16M binding. Furthermore, we report the structure of the FACT heterodimerization domain that connects FACT to replicative polymerases. Our results show that Spt16M makes several interactions with histones, which we suggest allow the module to invade the nucleosome gradually and block the strongest interaction of H2B with DNA. FACT would thus enhance 'nucleosome breathing' by re-organizing the first 30 base pairs of nucleosomal histone-DNA contacts. Our snapshot of the engagement of the chaperone with H2A-H2B and the structures of all globular FACT domains enable the high-resolution analysis of the vital chaperoning functions of FACT, shedding light on how the complex promotes the activity of enzymes that require nucleosome reorganization.
Pubmed